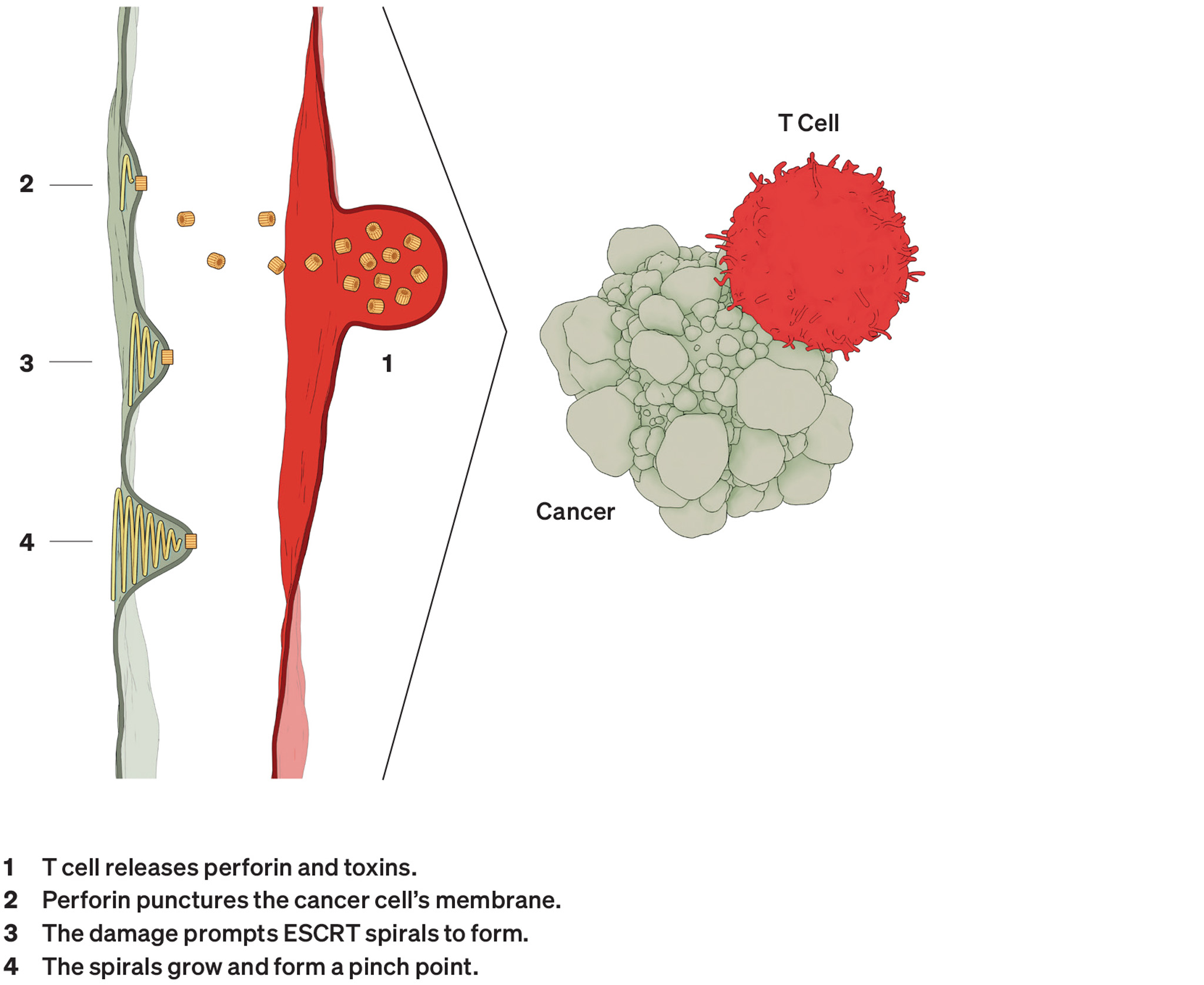
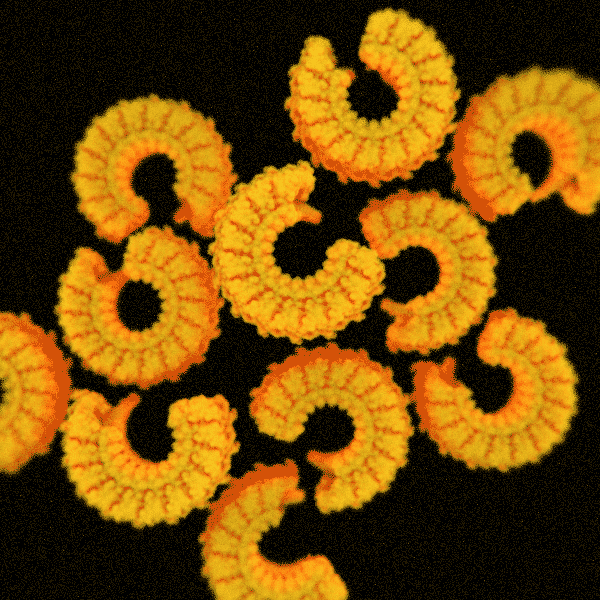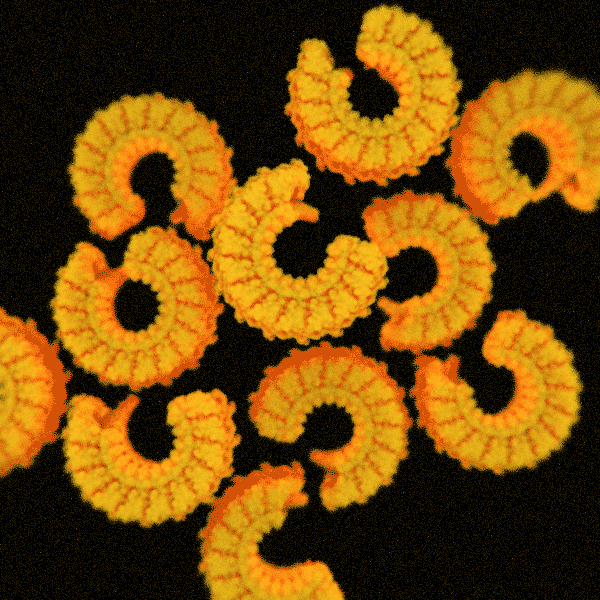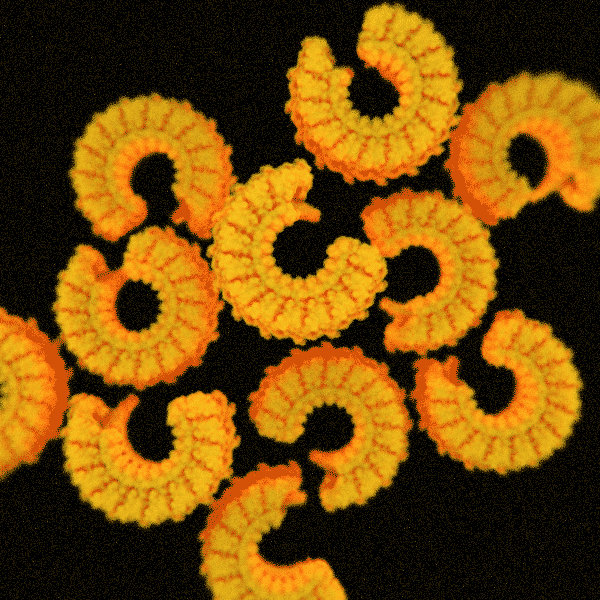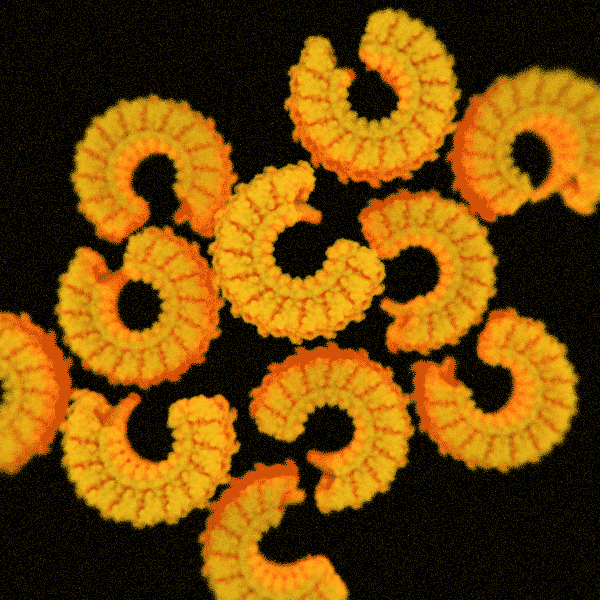Co-opted by Cancer
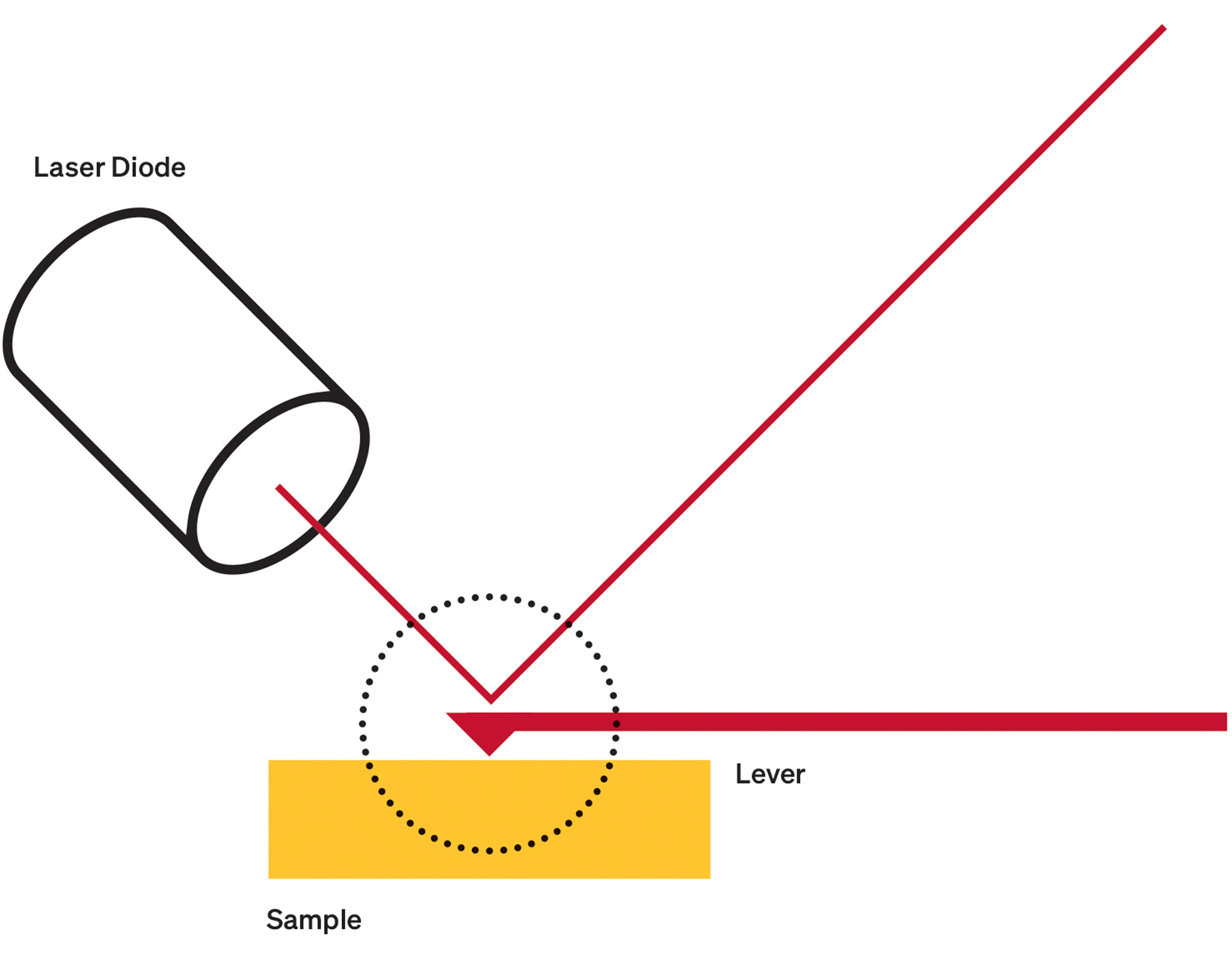
Dr. Scheuring’s lab has applied HS-AFM to numerous membrane protein structures, including two that other research suggests can face off against each other in cancer.
Perforin
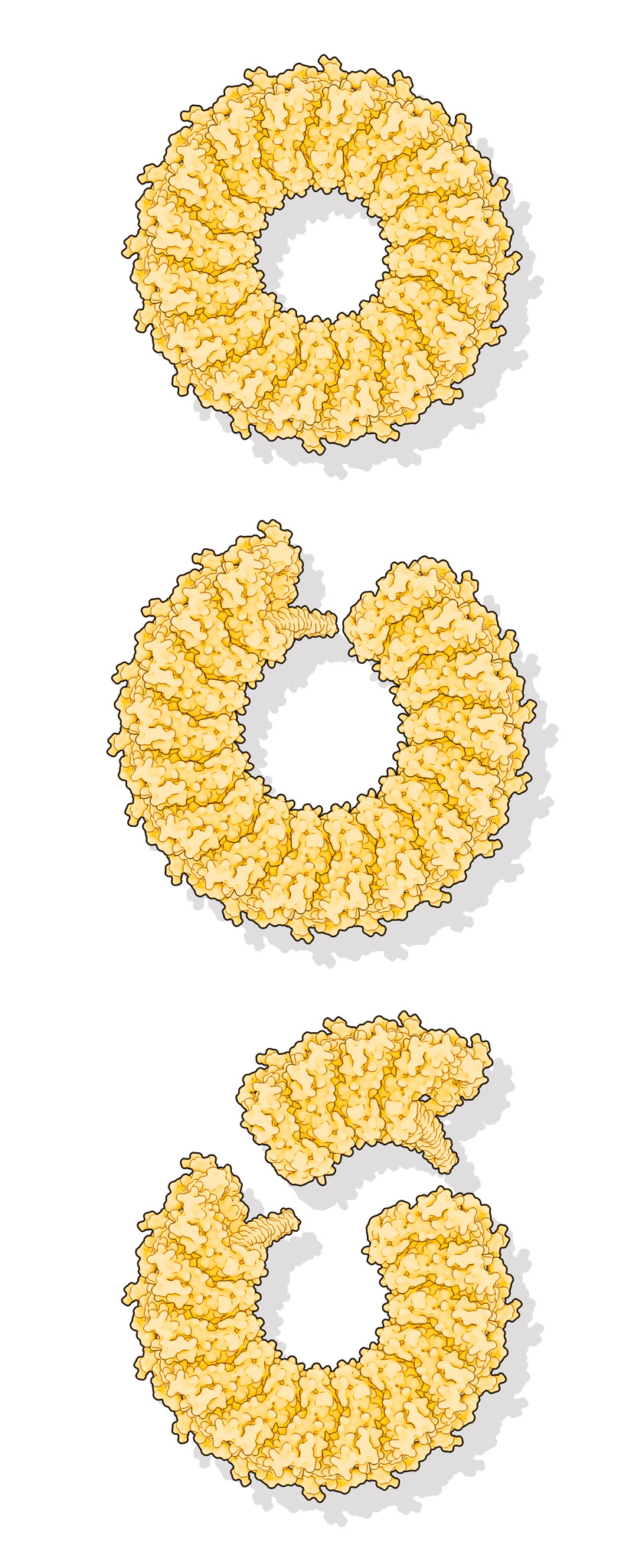
As its name suggests, the first one, perforin, pokes holes. Produced by the immune system to eliminate bacteria and infected or malignant cells, doughnut-shaped perforin molecules first land on their target. They then reconfigure to puncture that cell’s membrane, opening a passageway for toxic enzymes to finish the destruction of the target cell.
Cryo-electron microscopy studies had already determined the detailed structures for perforin just before and after it pokes through a membrane, but no one knew what happened in between. With HS-AFM, the group documented this transition, which traverses the round molecule section by section, like dominoes falling clockwise. It often stops before completing the circle. An incomplete ring appears adequate to do the job.
ESCRT
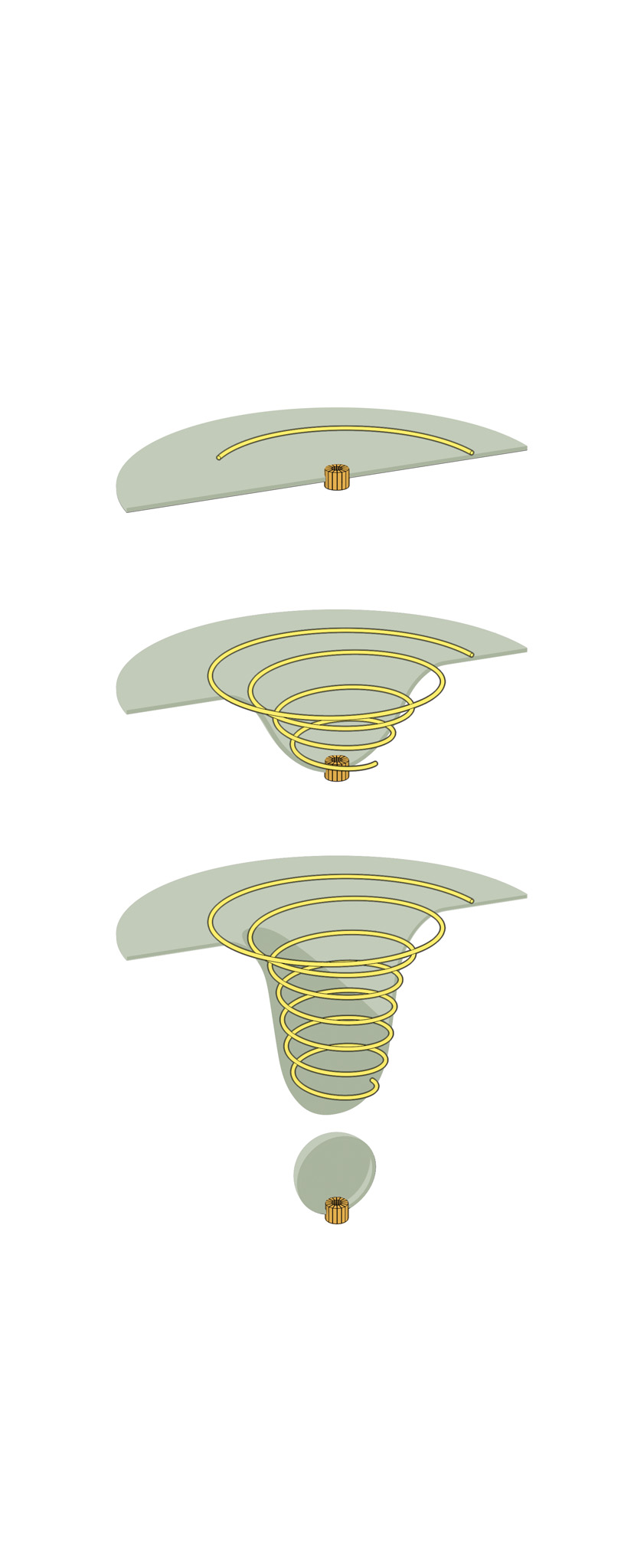
The second structure consists of elegant spirals built by the inelegantly named endosomal sorting complex required for transport system, otherwise known as ESCRT. As the name suggests, it helps cells sort and transport waste. ESCRT also enables them to sever connections and repair wounds.
HS-AFM recordings have tracked the formation of these structures, leading researchers to suggest they behave like springs, with the spiral compressing inward as the filament grows. Ultimately, the pressure forces its inner rings to pop outward.
Just as it protects the membranes of healthy cells, the spirals come to the aid of malignant ones. A study led by researchers with the company Genentech recently found that when under attack by perforin, tumor cells heal their wounds with ESCRT. This discovery suggests ESCRT could, perhaps, serve as a target for cancer drugs that aid the immune system.
A Way In
There is a catch, however. Any therapy that disrupts these wound-healing spirals in cancer risks interfering with them in healthy cells. Should anyone follow up on the possibility of an ESCRT-jamming drug, HS-AFM would provide a straight-forward method to examine how it affects the formation of these spirals on the surface of malignant or normal cells, Dr. Scheuring says.
In general, membrane proteins have proven themselves immensely important to treating disease. They are estimated to account for the majority of molecules targeted by medications.
For Dr. Scheuring, this bias is intuitive. “In order to have an effect on our being, drugs must pass through the membrane barrier, generally by interacting with these proteins,” he says. “In some ways, all of what we are is inside cells.”