Microglia
Features
How the brain’s less celebrated cells may drive Alzheimer’s disease and other dementias.
Talkative neurons, those long-armed nerve cells that signal one another, get credit for much of what the brain accomplishes and blame when things go wrong. Increasingly, however, researchers are focusing on their counterparts — the cells that quietly protect the brain and ensure it functions properly. New recognition of their roles in cognitive disorders is leading to potential new treatment strategies.
Named for their stellar shape, astrocytes ensure neurons develop and work properly. For example, a single one can extend into hundreds of thousands of synapses, the communication hubs between neurons, regulating the transmission of signals that travel across these junctions.
“Neurons alone cannot function normally. There would be no brain without astrocytes,” says Dr. Anna Orr, an assistant professor of neuroscience and the Nan and Stephen Swid Assistant Professor of Frontotemporal Dementia Research, who became fascinated with astrocytes as a postdoctoral fellow.
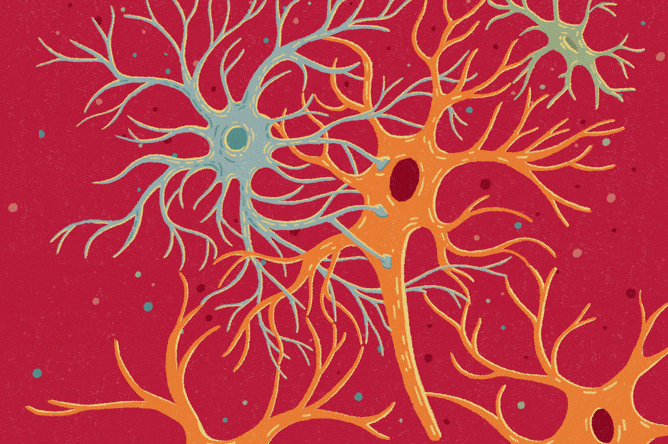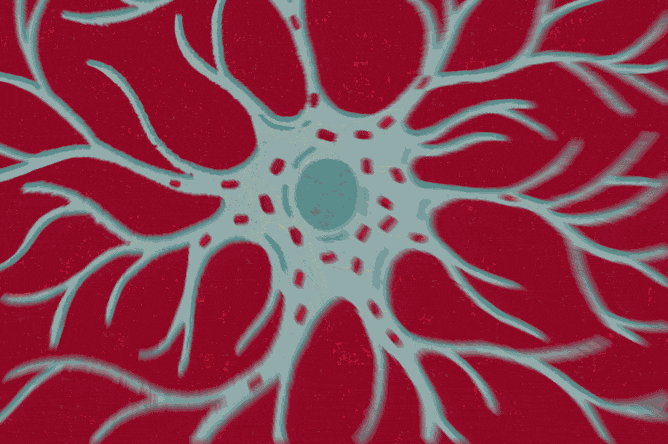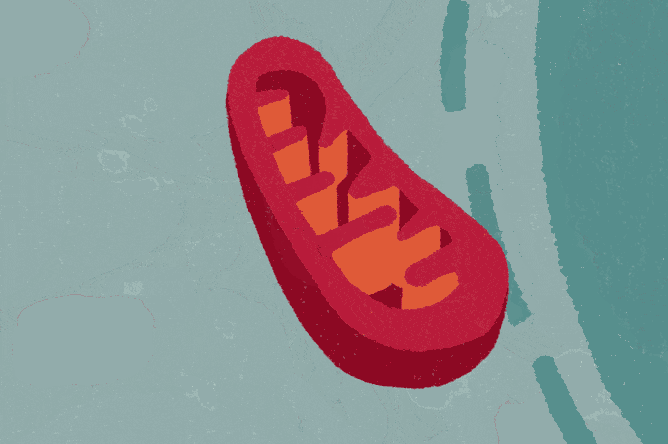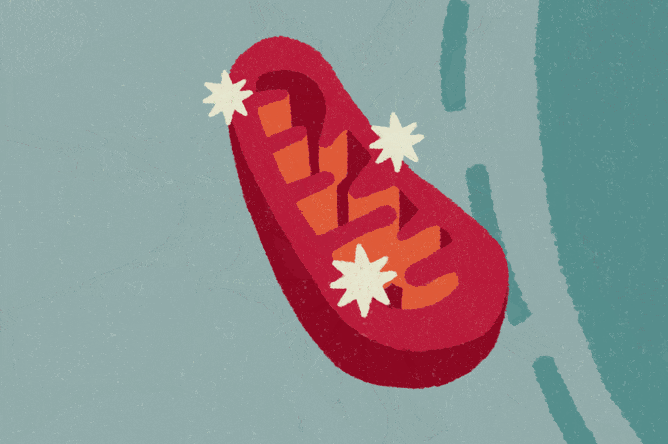
Meanwhile, Dr. Adam Orr, her husband and an assistant professor of research in neuroscience, focuses on mitochondria, specialized parts within cells responsible for repackaging energy from food into a readily usable form, among other crucial tasks. “Just as astrocytes are integral to brain function, mitochondria are integral to cell function,” he says.
Under normal circumstances, mitochondria produce low amounts of highly reactive, oxygen-containing compounds, such as superoxide (O2-) and hydrogen peroxide (H2O2). However, they generate more of these compounds in the presence of tau tangles, inflammatory signals from microglia, and other changes that occur in Alzheimer’s disease and frontotemporal dementia. This increase sets off an internal alarm within the astrocytes, causing these cells to behave differently and disrupt neuronal function, according to Dr. Anna Orr.
The Orrs’ work suggests that the alarmed astrocytes can aggravate disease, including by worsening some of the very factors that instigated their transformation, such as tau tangles and brain inflammation.
Not only are these reactive compounds byproducts of normal processes, evidence also suggests that, at regular levels, they are required for healthy cell function. So, eliminating them entirely may not be the best option. Instead, the Orrs are developing precise modulators that block the excess production at its source, within particular sites in mitochondria, without interfering with these organelles’ ability to supply energy and carry out other functions.

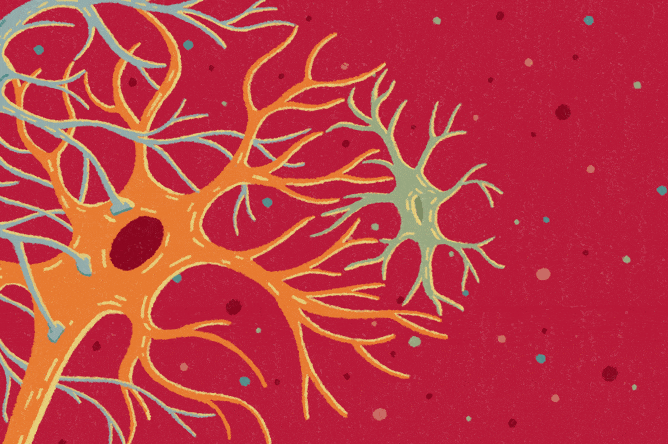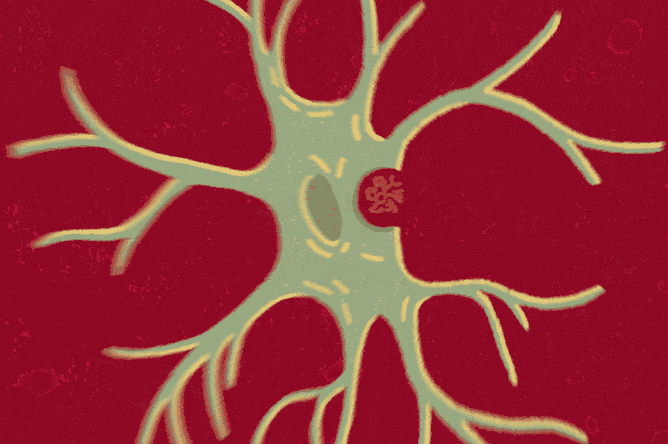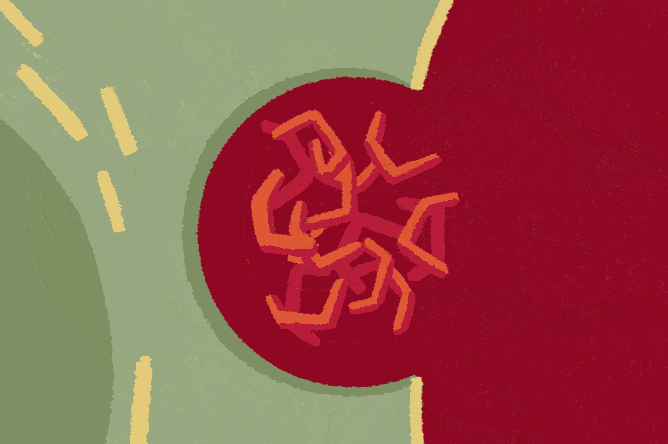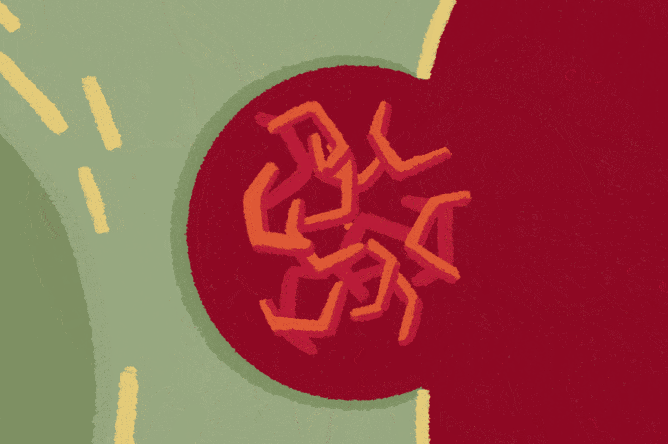
Immune cells unique to the brain, microglia keep the brain safe and in working order by consuming and digesting any potential threat, whether a pathogen or piece of debris. Evidence suggests that these cells also contribute to cognitive decline.
“A lot of genetic mutations that affect microglia also lead to a higher risk of frontotemporal dementia and Alzheimer’s disease” says Dr. Li Gan, director of the Helen and Robert Appel Alzheimer’s Disease Research Institute, professor of neuroscience, and the Burton P. and Judith B. Resnick Distinguished Professor in Neurodegenerative Diseases. “We want to find out why.”
This quest has led her to tau, a versatile, fiber-like protein found within cells, most notably in neurons. In dementia, tau misbehaves, forming tangles. As these tangles spread through the brain, patients’ conditions deteriorate. Dr. Gan’s research suggests that the microglia may inadvertently propel this progression.
When microglia encounter tau tangles floating about in the space between cells, the tangles activate an immunological switch within them. This pathway, known as NF-kB, tells the microglia it’s time to eat. These cells then rearrange their boundaries to surround and consume the tau tangles.
However, in doing so, microglia appear to cause more harm than good, Dr. Gan and her colleagues have found. Rather than fully breaking down their targets, microglia spit out toxic bits of tau. Other brain cells pick up these pieces, which seed new tangles. When ejected by the cells, these new tangles only further activate the microglia. Thus, a vicious cycle develops, fueling the tau tangles’ spread through the brain.
By shutting down NF-kB, Dr. Gan’s team interfered with the spread of tau tangles in mice. However, this isn’t a realistic strategy for therapies because, without NF-kB, microglia become paralyzed and cannot defend the brain. What’s more, the loss of this pathway could also potentially impair the immune system in the rest of the body.
Subsequent research, however, has led to a more promising route: a branch of the NF-kB pathway activated by an enzyme called cGAS. Under normal circumstances, it responds only to viral infections. In dementia, however, it picks up on cellular damage caused by tau tangles. In experiments, researchers found that shutting off cGAS in microglia had a protective effect on neurons and could restore memory in mice.
In terms of potential therapies, “NF-kB is a sledgehammer, cGAS is a scalpel,” Dr. Gan says.
Illustrations: Yukai Du